Single- and Multiple-Dose Study To Determine the Safety, Tolerability, Pharmacokinetics, and Food Effect of Oral MRX-I versus Linezolid in Healthy Adult Subjects | Antimicrobial Agents and Chemotherapy

CR TidBit – Single Ascending Dose to Multiple Ascending Dose. | AuroBlog | Clinical Research Blog | Aurous HealthCare CRO, India

Dr. Martha Gulati ♥️🫀❤️🩹🇨🇦 в Twitter: „#APOLLO #ACC22 #LBCT Phase 1 trial by Dr. Nissen 🫀⬆️ Lp(a) highly prevalent 🫀Those w/o ASCVD: siRNA effect on Lp(a) at 150d 💥Dose dependent ⬇️ in
Single and Multiple Dose Pharmacokinetics, Pharmacodynamics and Safety of the Novel Lipoprotein-Associated Phospholipase A2 Enzyme Inhibitor Darapladib in Healthy Chinese Subjects: An Open Label Phase-1 Clinical Trial | PLOS ONE
![PDF] Single and Multiple Ascending-dose Studies of Oral Delafloxacin: Effects of Food, Sex, and Age. | Semantic Scholar PDF] Single and Multiple Ascending-dose Studies of Oral Delafloxacin: Effects of Food, Sex, and Age. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/168f1250036d7761525361015074a1a817a3139b/7-Figure1-1.png)
PDF] Single and Multiple Ascending-dose Studies of Oral Delafloxacin: Effects of Food, Sex, and Age. | Semantic Scholar
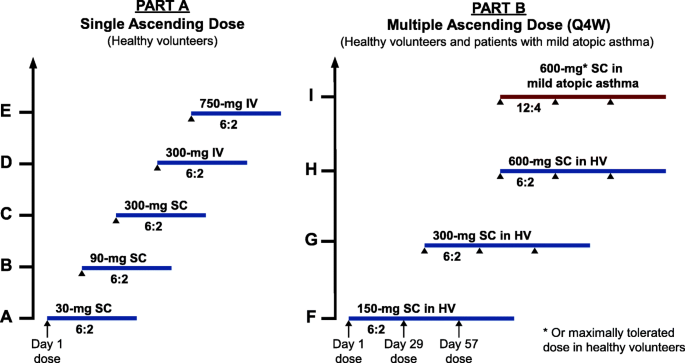
A phase I, randomized, observer-blinded, single and multiple ascending-dose study to investigate the safety, pharmacokinetics, and immunogenicity of BITS7201A, a bispecific antibody targeting IL-13 and IL-17, in healthy volunteers | BMC Pulmonary

Single and Multiple Ascending-dose Studies of Oral Delafloxacin: Effects of Food, Sex, and Age - ScienceDirect

Design of a single-dose study, b multiple-dose study, and c meals and... | Download Scientific Diagram
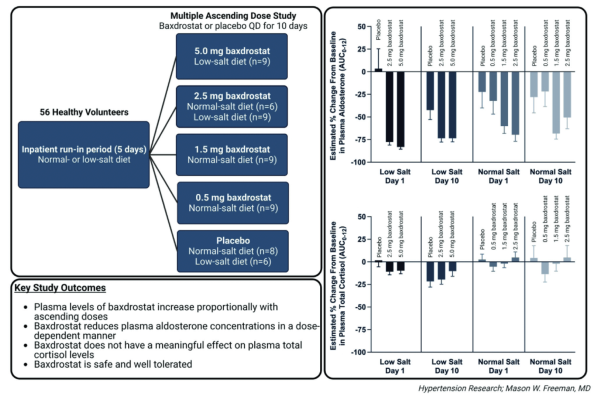
CinCor Pharma Announces Publication of Phase 1 Multiple Ascending Dose Study Data in Hypertension Research | DAIC
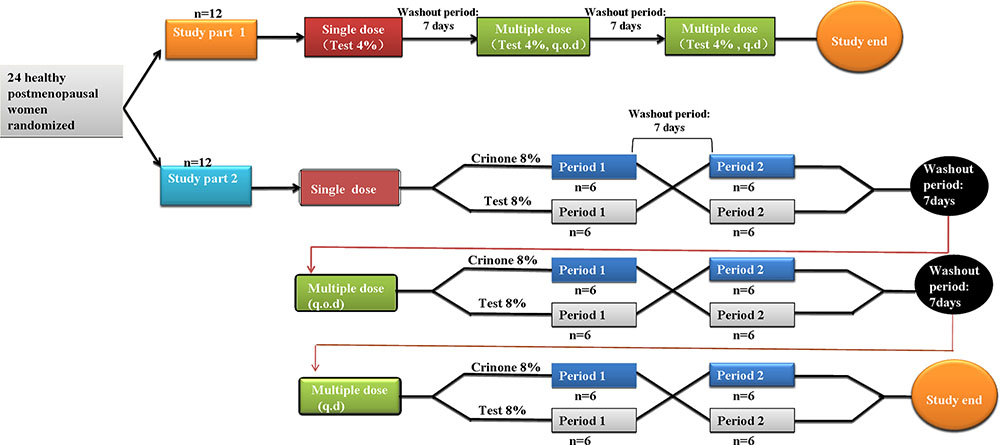
Frontiers | Pharmacokinetic Properties of Three Forms of Vaginal Progesterone Administered in Either Single Or Multiple Dose Regimen in Healthy Post-menopausal Chinese Women

Design of a single-dose study, b multiple-dose study, and c meals and... | Download Scientific Diagram

Pharmacokinetics of RP5063 Following Single Doses to Normal Healthy Volunteers and Multiple Doses Over 10 Days to Stable Schizophrenic Patients - Cantillon - 2018 - Clinical and Translational Science - Wiley Online Library