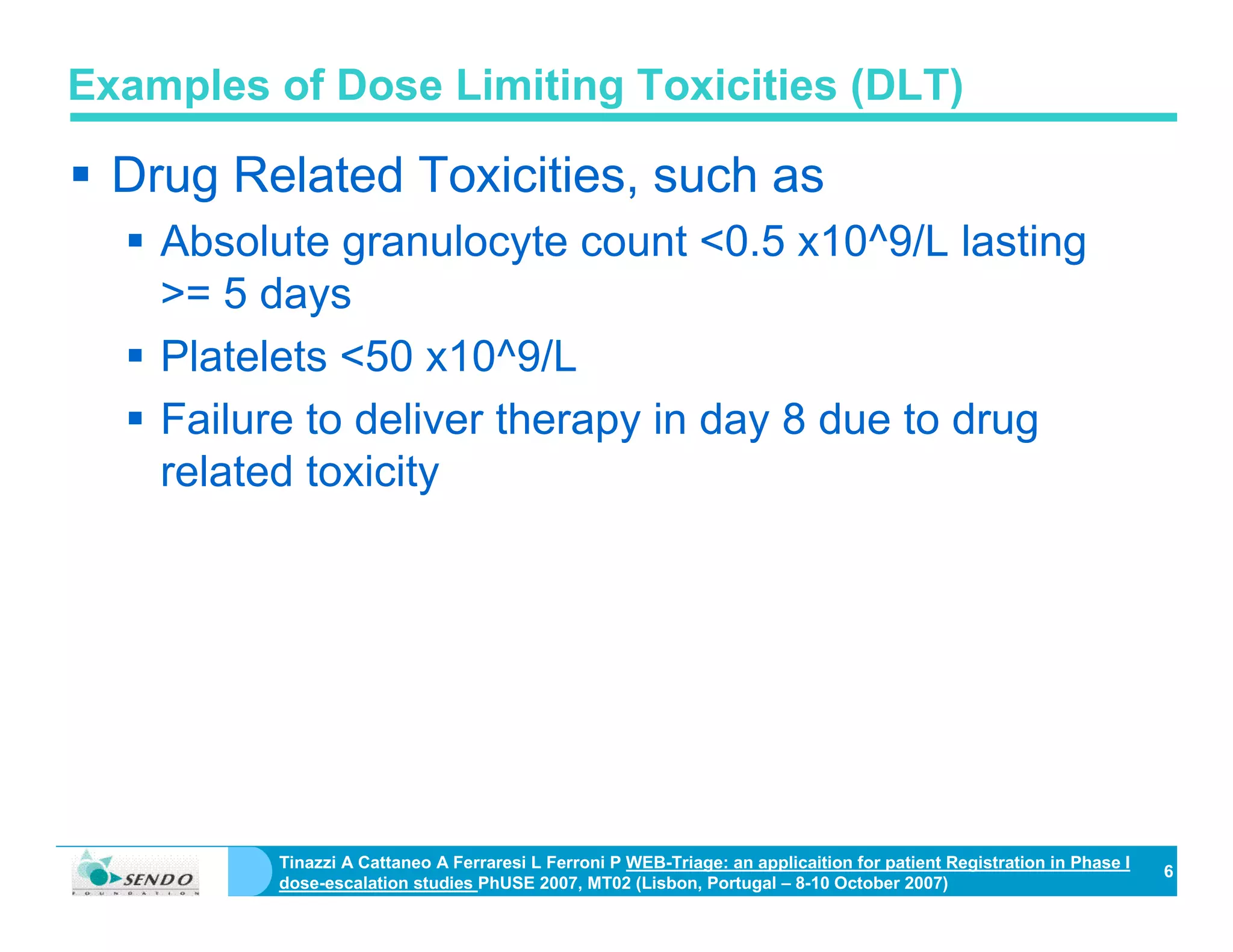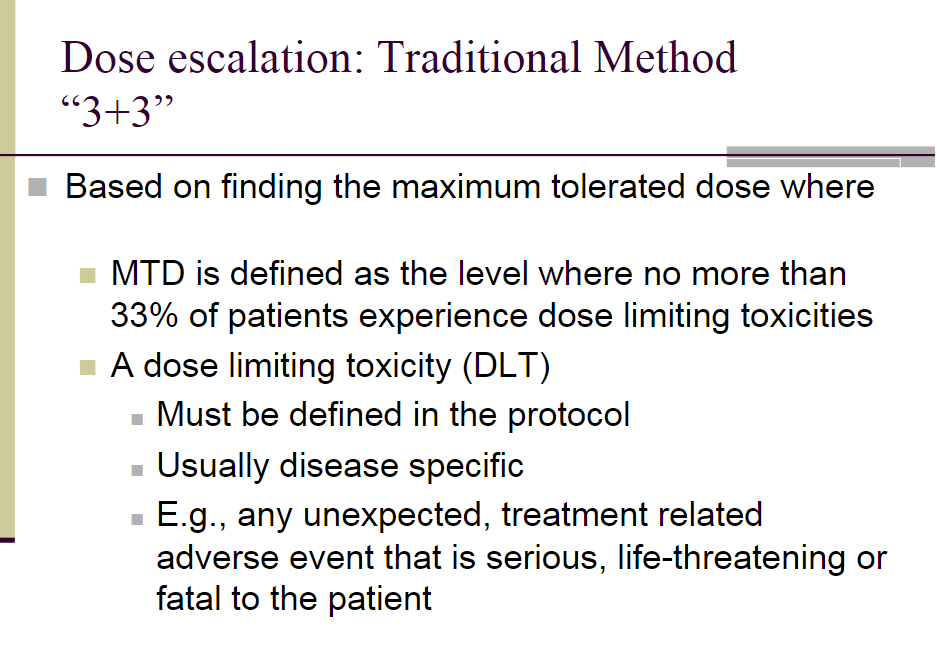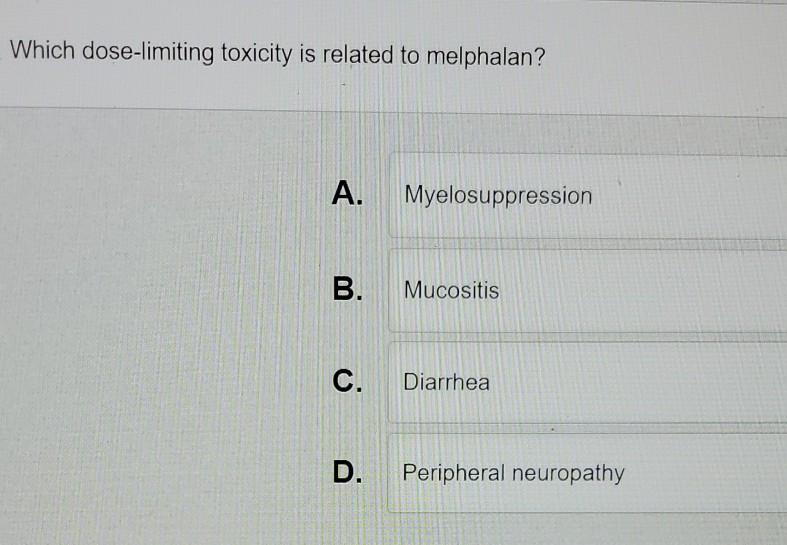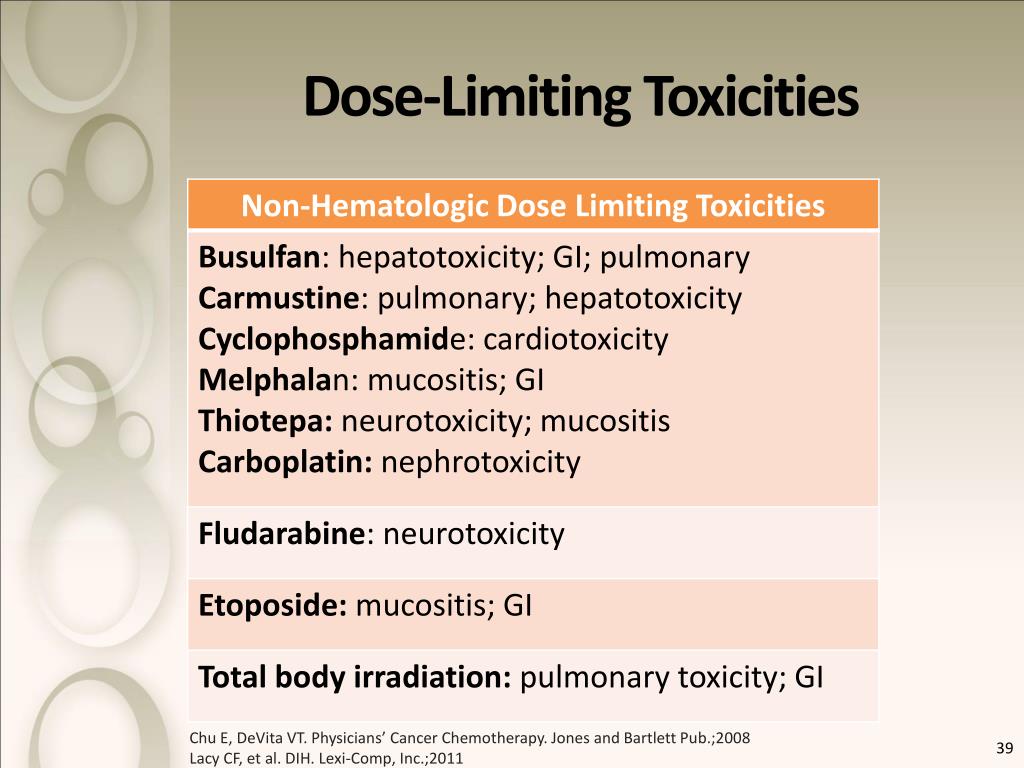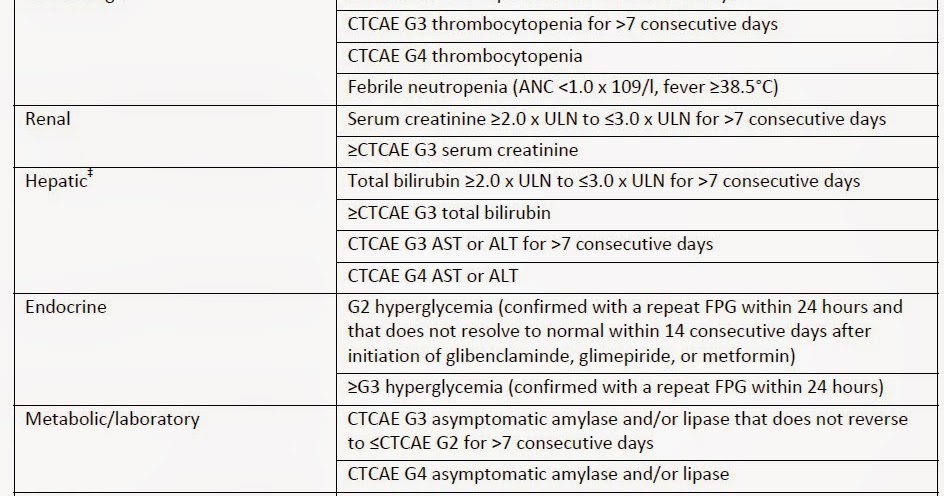
On Biostatistics and Clinical Trials: Dose Limiting Toxicity (DLT) and Common Toxicity Criteria (CTC) / Common Terminology Criteria for Adverse Events (CTCAE)
TAT 2011 presentation: Definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents

Targeting synovial fibroblast proliferation in rheumatoid arthritis (TRAFIC): an open-label, dose-finding, phase 1b trial - The Lancet Rheumatology

Trends in the characteristics, dose-limiting toxicities and efficacy of phase I oncology trials: The Cancer Research UK experience - ScienceDirect

Single-arm, open-label, dose-escalation phase I study to evaluate the safety of a herbal medicine SH003 in patients with solid cancer: a study protocol | BMJ Open
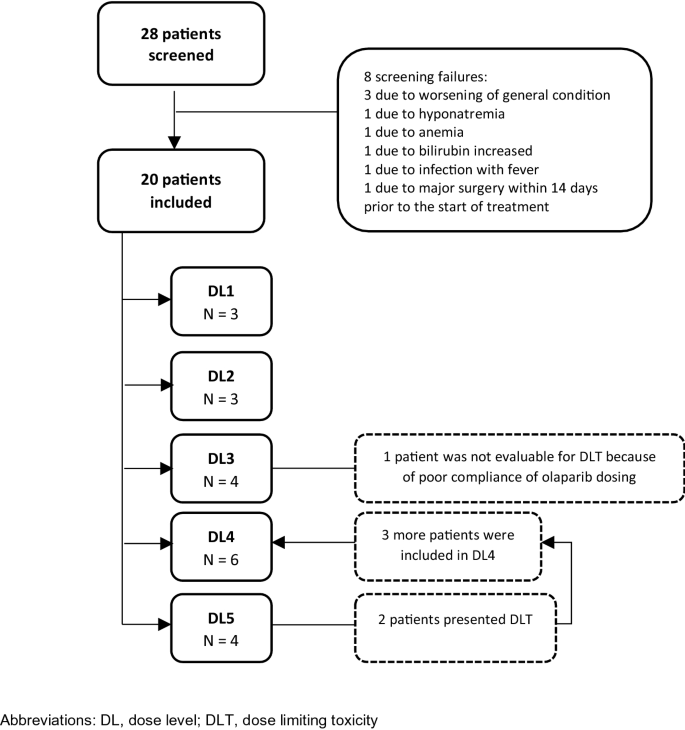
A phase I dose-finding, pharmacokinetics and genotyping study of olaparib and lurbinectedin in patients with advanced solid tumors | Scientific Reports

Innovative design for a phase 1 trial with intra-patient dose escalation: The Crotoxin study. - Abstract - Europe PMC
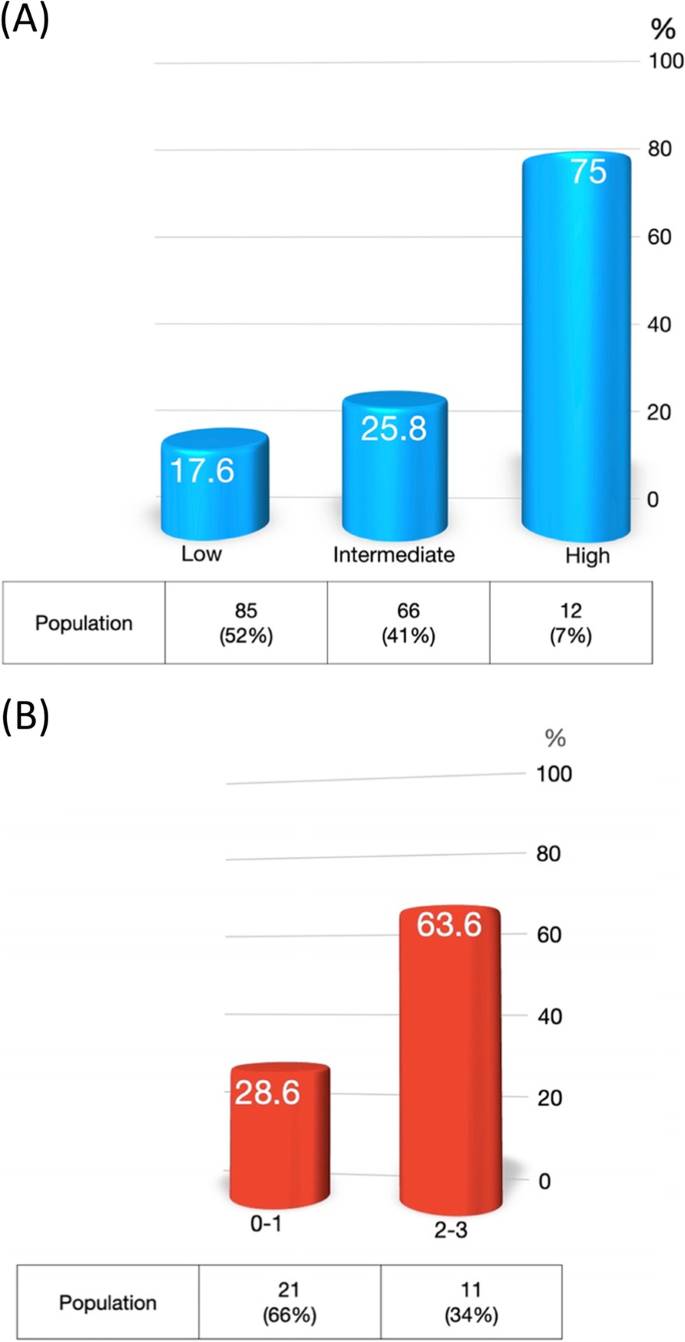
Chemotherapy dose per kilogram lean body mass increased dose-limiting toxicity event in male head and neck cancer with taxane and platinum-based induction therapy | BMC Cancer | Full Text

Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents – Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group

Illustration of the chronic dose-limiting toxicity (DLT) concept. (*)... | Download Scientific Diagram