A facile approach to prepare Bi(OH) 3 nanoflakes as high-performance pseudocapacitor materials - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C5NJ01017A

Biocompatible Bi(OH)3 nanoparticles with reduced photocatalytic activity as possible ultraviolet filter in sunscreens - ScienceDirect

Bi2(SO4)3+NH4OH=Bi(OH)3+(NH4)2SO4 balance the chemical equation @mydocumentary838. bi2(so4)3+nh4oh= - YouTube
![Complete and balance the following equation (a). Bi(OH)3+SnO2^(2-)toBi+SnO3^(2-) (b). [Fe(CN)6]^(3-)+Cr2O7^(2-)toFe^(3+)+Cr^(3+)+CO2+NO3^(ɵ) (c). VtoPH3+P4H2 (d). P2H4toPH3+P4H2 (e). Ca3(PO4)2+SiO2+CtoCaSiO3+P4+CO Complete and balance the following equation (a). Bi(OH)3+SnO2^(2-)toBi+SnO3^(2-) (b). [Fe(CN)6]^(3-)+Cr2O7^(2-)toFe^(3+)+Cr^(3+)+CO2+NO3^(ɵ) (c). VtoPH3+P4H2 (d). P2H4toPH3+P4H2 (e). Ca3(PO4)2+SiO2+CtoCaSiO3+P4+CO](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/11032936_web.png)
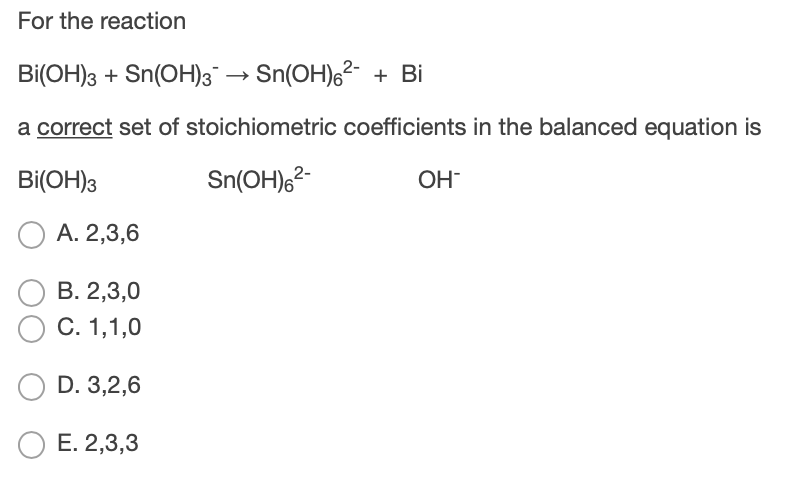
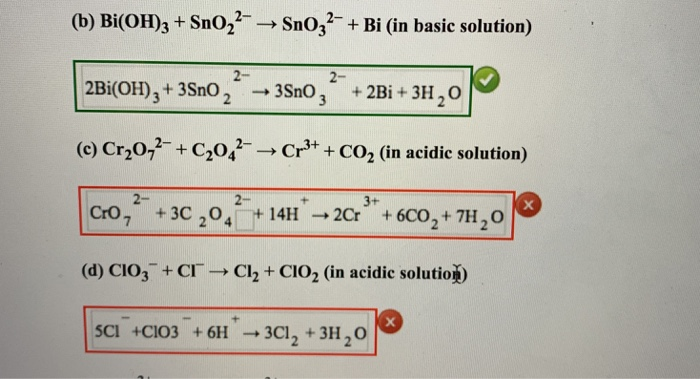
Complete and balance the following equation (a). Bi(OH)3+SnO2^(2-)toBi+SnO3^(2-) (b). [Fe(CN)6]^(3-)+Cr2O7^(2-)toFe^(3+)+Cr^(3+)+CO2+NO3^(ɵ) (c). VtoPH3+P4H2 (d). P2H4toPH3+P4H2 (e). Ca3(PO4)2+SiO2+CtoCaSiO3+P4+CO

High toxicity of Bi(OH)3 and α-Bi2O3 nanoparticles towards malignant 9L and MCF-7 cells. | Semantic Scholar

Chapter 4Reactions in Aqueous Solutions. Some typical kinds of chemical reactions: 1.Precipitation reactions: the formation of a salt of lower solubility. - ppt download
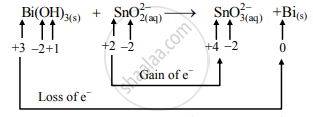
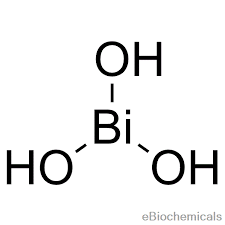
Balance the following redox equation by half-reaction method. Bi(OH)X3(s)+SnOX2(aq)2−⟶SnOX3(aq)2−+BiX(s)(basic) - Chemistry | Shaalaa.com
Balance the following redox equation by half reaction method : Bi(OH)3(s) + SnO^(2-)2(aq) ⟶ SnO^(2-)3(aq) + Bi(s) - Sarthaks eConnect | Largest Online Education Community





3.svg)